Before batteries lose power, fail suddenly, or burst into flames, they tend to produce faint sounds over time that provide a signature of the degradation processes going on within their structure. But until now, nobody had figured out how to interpret exactly what those sounds meant, and how to distinguish between ordinary background noise and significant signs of possible trouble.
Now, a team of researchers at MIT’s Department of Chemical Engineering have done a detailed analysis of the sounds emanating from lithium ion batteries, and has been able to correlate particular sound patterns with specific degradation processes taking place inside the cells. The new findings could provide the basis for relatively simple, totally passive and nondestructive devices that could continuously monitor the health of battery systems, for example in electric vehicles or grid-scale storage facilities, to provide ways of predicting useful operating lifetimes and forecasting failures before they occur.
The findings were reported Sept. 5 in the journal Joule, in a paper by MIT graduate students Yash Samantaray and Alexander Cohen, former MIT research scientist Daniel Cogswell PhD ’10, and Chevron Professor of Chemical Engineering and professor of mathematics Martin Z. Bazant.
“In this study, through some careful scientific work, our team has managed to decode the acoustic emissions,” Bazant says. “We were able to classify them as coming from gas bubbles that are generated by side reactions, or by fractures from the expansion and contraction of the active material, and to find signatures of those signals even in noisy data.”
Samantaray explains that, “I think the core of this work is to look at a way to investigate internal battery mechanisms while they’re still charging and discharging, and to do this nondestructively.” He adds, “Out there in the world now, there are a few methods that exist, but most are very expensive and not really conducive to batteries in their normal format.”
To carry out their analysis, the team coupled electrochemical testing with recording of the acoustic emissions, under real-world charging and discharging conditions, using detailed signal processing to correlate the electrical and acoustic data. By doing so, he says, “we were able to come up with a very cost-effective and efficient method of actually understanding gas generation and fracture of materials.”
Gas generation and fracturing are two primary mechanisms of degradation and failure in batteries, so being able to detect and distinguish those processes, just by monitoring the sounds produced by the batteries, could be a significant tool for those managing battery systems.
Previous approaches have simply monitored the sounds and recorded times when the overall sound level exceeded some threshold. But in this work, by simultaneously monitoring the voltage and current as well as the sound characteristics, Bazant says, “We know that [sound] emissions happen at a certain potential [voltage], and that helps us identify what the process might be that is causing that emission.”
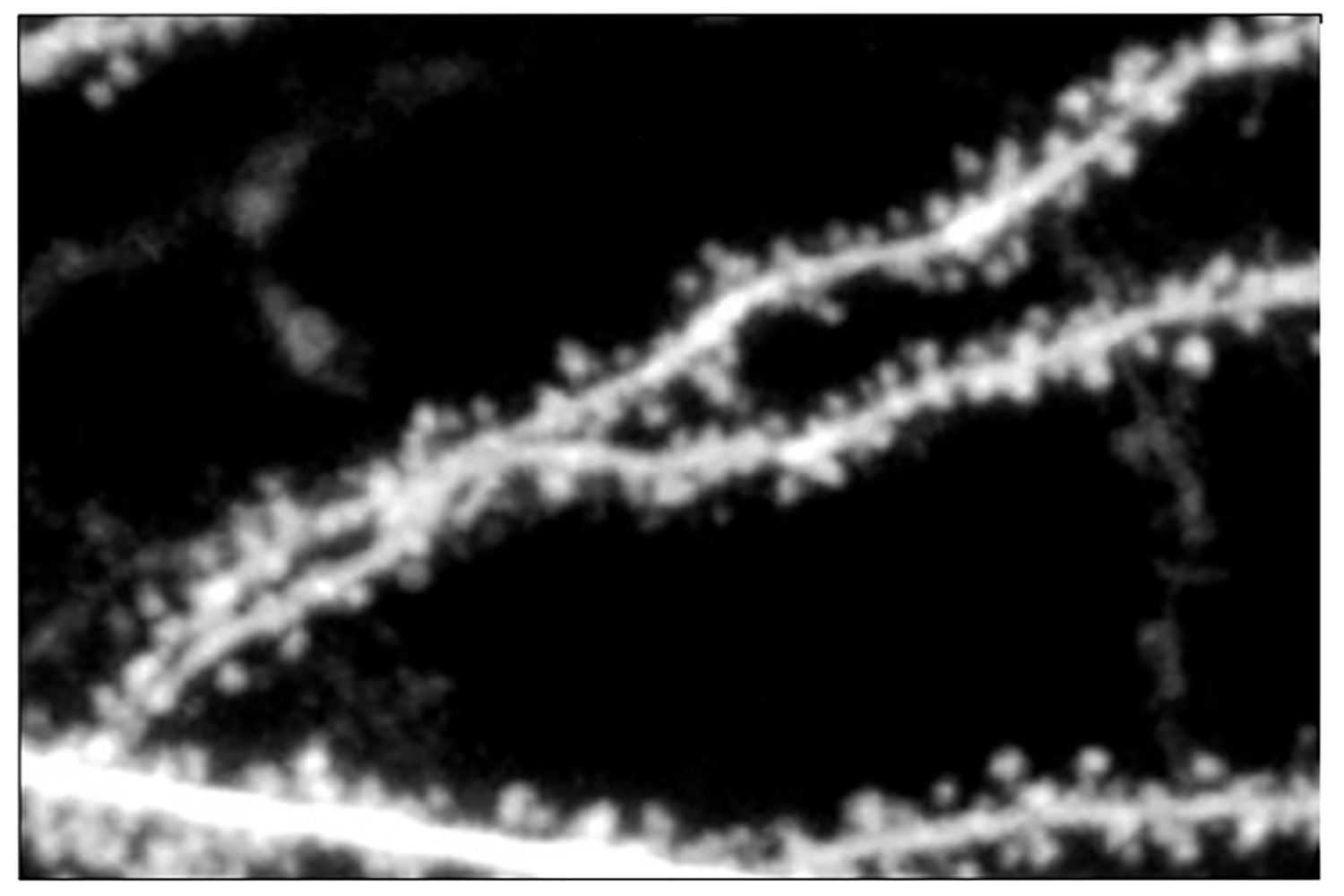
After these tests, they would then take the batteries apart and study them under an electron microscope to detect fracturing of the materials.
In addition, they took a wavelet transform — essentially, a way of encoding the frequency and duration of each signal that is captured, providing distinct signatures that can then be more easily extracted from background noise. “No one had done that before,” Bazant says, “so that was another breakthrough.”
Acoustic emissions are widely used in engineering, he points out, for example to monitor structures such as bridges for signs of incipient failure. “It’s a great way to monitor a system,” he says, “because those emissions are happening whether you’re listening to them or not,” so by listening, you can learn something about internal processes that would otherwise be invisible.
With batteries, he says, “we often have a hard time interpreting the voltage and current information as precisely as we’d like, to know what’s happening inside a cell. And so this offers another window into the cell’s state of health, including its remaining useful life, and safety, too.” In a related paper with Oak Ridge National Laboratory researchers, the team has shown that acoustic emissions can provide an early warning of thermal runaway, a situation that can lead to fires if not caught. The new study suggests that these sounds can be used to detect gas generation prior to combustion, “like seeing the first tiny bubbles in a pot of heated water, long before it boils,” says Bazant.
The next step will be to take this new knowledge of how certain sounds relate to specific conditions, and develop a practical, inexpensive monitoring system based on this understanding. For example, the team has a grant from Tata Motors to develop a battery monitoring system for its electric vehicles. “Now, we know what to look for, and how to correlate that with lifetime and health and safety,” Bazant says.
One possible application of this new understanding, Samantaray says, is “as a lab tool for groups that are trying to develop new materials or test new environments, so they can actually determine gas generation or active material fracturing without having to open up the battery.”
Bazant adds that the system could also be useful for quality control in battery manufacturing. “The most expensive and rate-limiting process in battery production is often the formation cycling,” he says. This is the process where batteries are cycled through charging and discharging to break them in, and part of that process involves chemical reactions that release some gas. The new system would allow detection of these gas formation signatures, he says, “and by sensing them, it may be easier to isolate well-formed cells from poorly formed cells very early, even before the useful life of the battery, when it’s being made,” he says.
The work was supported by the Toyota Research Institute, the Center for Battery Sustainability, the National Science Foundation, and the Department of Defense, and made use of the facilities of MIT.nano.